
S. Bisicchia, G. Bernardi, C. Tudisco
HYMOVIS® (HYADD®4, Fidia Farmaceutici Spa, Abano Terme, Italy) is a new hyaluronic acid (HA) based hydrogel for the treatment of osteoarthritis knee pain, which has improved shock absorbing and lubricating properties.*
This is a single-center, single-blind, prospective randomized controlled clinical study to compare clinical results and quality of life scores in patients with symptomatic knee osteoarthritis randomized to either HYMOVIS® or corticosteroid (CS). An additional analysis was conducted for safety and tolerability.
Patients were evaluated at baseline, 6, 12, 26 and 52 weeks post-injection.
Primary end point: WOMAC total score at 26 weeks;
Secondary end points: WOMAC total score, VAS for pain, and SF-36 score at any time point.
Results
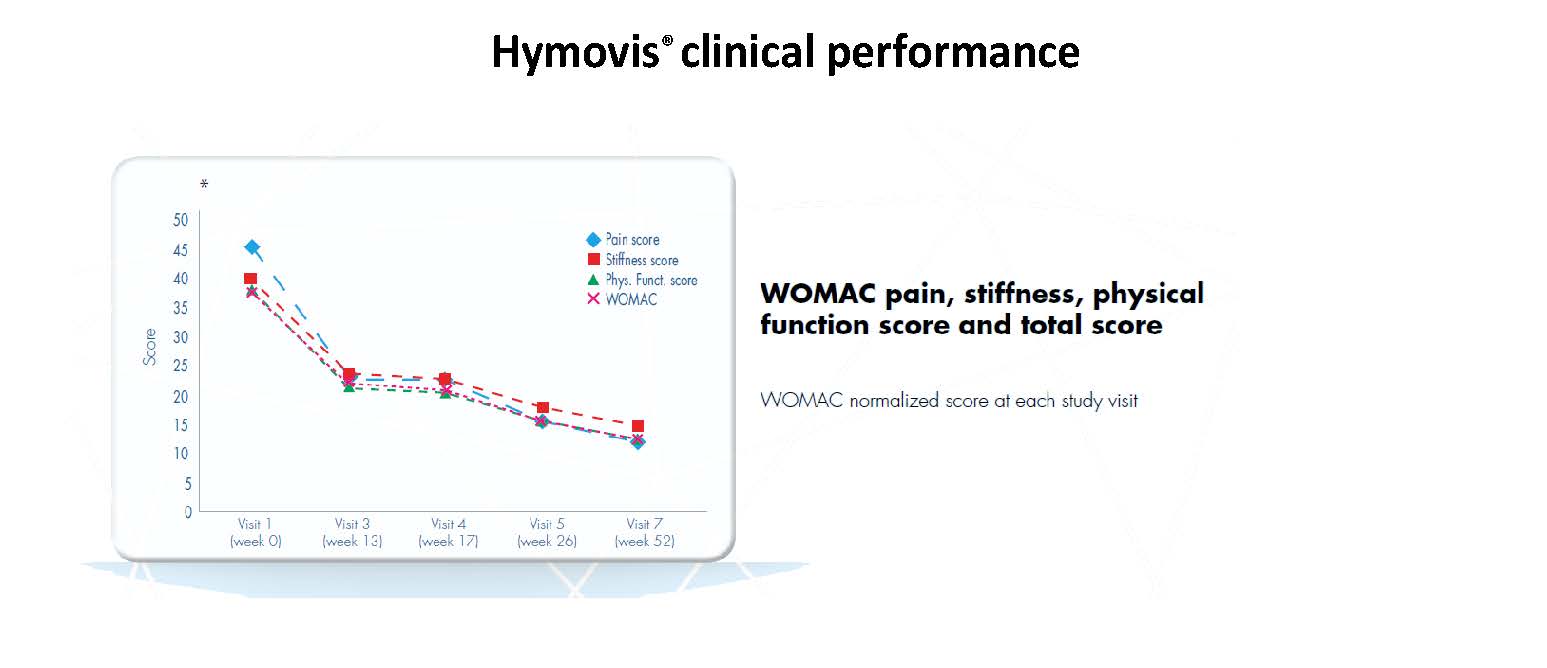
HYMOVIS® clinical performance
While patients in both groups obtained good results in the short term (6 weeks), patients in the HYMOVIS® group obtained better results at 26 weeks in terms of knee function, WOMAC total scores, VAS for pain, and quality of life as reported by SF-36 scores (p<0.0001). HYMOVIS® demonstrated an excellent safety profile with no significant difference in reported adverse events between the two groups in the study.
HYMOVIS®-treated patients had significantly greater improvement in total WOMAC scores, VAS for pain scores, and SF-36 scores at 26 weeks
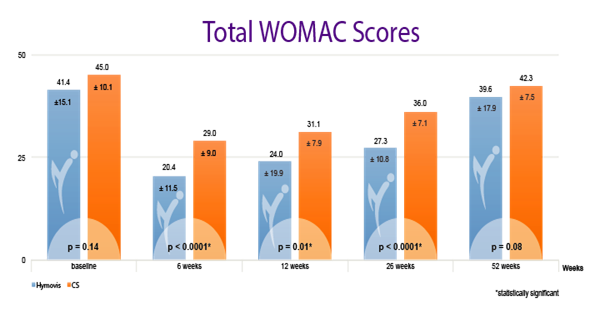
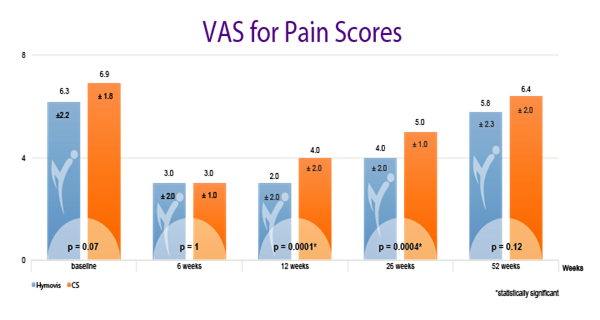
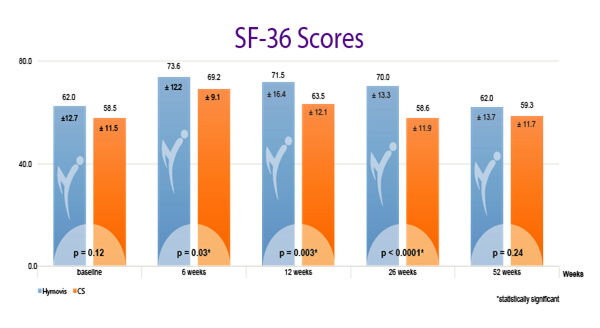
Efficacy outcomes in patients treated with 2 weekly IA injections of either HYMOVIS® (HYADD®4) or corticosteroid: Total WOMAC Scores; VAS for Pain Scores; and quality of life as shown by patient responses on the SF-36 questionnaire.
*Data on file

New data presented March 22, 2017 at the Orthopedic Research Society Annual Meeting highlights the superior lubricating properties of HYMOVIS® against all other competitor HA products evaluated.
HYMOVIS® has exceptional lubricating properties in moving cartilage to a low-friction regime. The study provides a unifying framework to evaluate the potential clinical efficacy of injectable hyaluronic acid therapies.
Fidia Farmaceutici S.p.A., a world leader in the research, development and manufacturing of hyaluronic acid (HA) - based products and its wholly owned subsidiary, FIDIA PHARMA USA INC., announces new data were presented at the Annual Meeting of the Orthopaedic Research Society (ORS) in San Diego, CA, March 19-22, 2017.
Scientists from Fidia Farmaceutici S.p.A. and the research group led by Dr. Lawrence Bonassar, Professor at the Meinig School of Biomedical Engineering at Cornell University evaluated the in vitro rheological and tribological properties of six commercial HA products used for the treatment of pain in osteoarthritis (OA) of the knee. “In our research, we demonstrated that there are clear differences in the lubricating abilities of this cohort of HA products. HYMOVIS® is an exceptional viscous lubricant in moving cartilage to a low-friction regime, having superior biomechanical lubricating properties amongst all the HAs evaluated.” In addition, the study approach offers a novel method to evaluate the potential clinical efficacy of injectable hyaluronic acid therapies that may be more sensitive than rheological measurements alone.
This new data were presented in the late breaking session of the Annual Meeting of the Orthopaedic Research Society (ORS) on Wednesday, March 22, 2017 (http://www.ors.org/Transactions/63/2374.pdf). (Pending publication).
HYMOVIS® is a highly viscoelastic, non-crosslinked hydrogel bioengineered using a proprietary process that increases lubrication and shock absorption properties. This results in a natural hyaluronan similar to the hyaluronan found in the synovial fluid present in human joints. The formulation allows this unique molecule to recover its original structure, even after repetitive mechanical stress. Due to reversible hydrophobic interactions, the non-crosslinked HYMOVIS® has increased elasticity, viscosity and residence time in the joint. Its unique molecular structure results in enhanced biomechanical properties and long-lasting efficacy, all in a convenient two-dose regimen.
F. Benazzo, L. Perticarini, A. Padolino, A. Castelli, P. Gifuni, M. Lovato, C. Manzini, N. Giordan
Viscosupplementation with intra-articular injection of hyaluronic acid (HA) is widely used for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy or simple analgesics.
HYMOVIS® is a newly marketed HA manufactured by Fidia Farmaceutici S.p.A., Abano Italy, and distributed by its wholly owned subsidiary, FIDIA PHARMA USA INC., Parsippany, NJ. HYMOVIS® consists of an 8mg/mL hydrogel formulation of HYADD®4, a unique molecule, which is not chemically crosslinked and has a low degree of modification that increases viscosity, elasticity and joint residence time (HYMOVIS package insert).
This is a prospective, multicentre, open label, 12 months follow-up, clinical study to evaluate the long-term benefits of 2 cycles of treatment with 2x3 ml intra-articular injections of 8 mg/ml of HYMOVIS® in the treatment of knee OA (n=49 outpatients). Each injection cycle was given at weekly intervals for two weeks, repeated at 6 months.
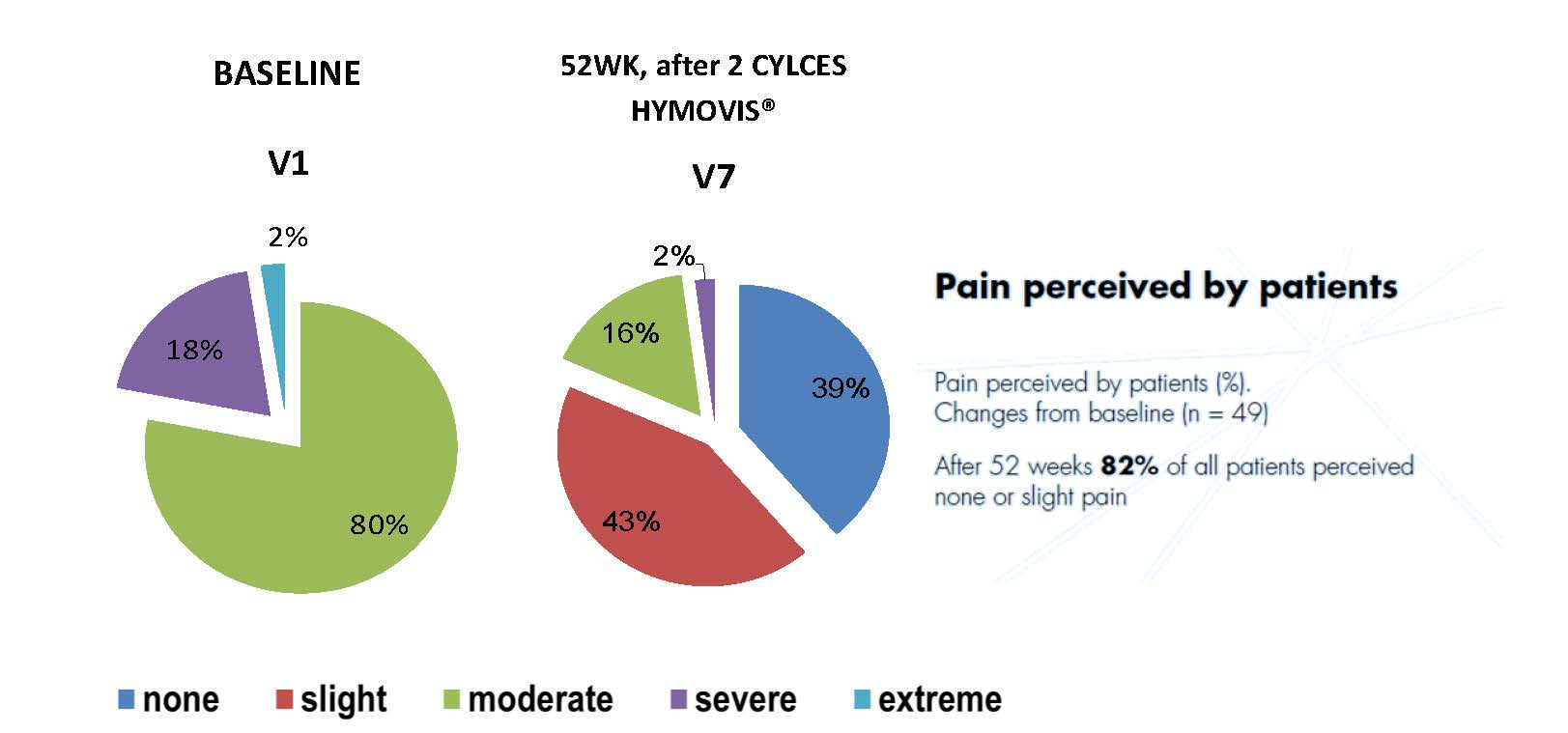
Results
Clinical performance and safety of HYMOVIS® in the treatment of pain due to knee OA, demonstrated in previous studies has been confirmed by the results of this study.

A breakthrough in the treatment of osteoarthritis (OA) of the knee, HYMOVIS® is helping active patients everywhere write their own story about moving past pain and back to the things they love in life.
Take a moment to learn about how a simple, two-injection regimen can be a convenient, efficient treatment of OA for your patients.

|
Fidia offers COMPREHENSIVE SUPPORT for your patients and your practice The HYMOVIS® Support Hotline*: 1-866-HYMOVIS (1-866-496-6847) or www.HYMOVISonline.com
|
| No minimum order quantity | |
| Low thresholds for contract pricing | |
| Easy online ordering | |
| Each carton contains two blister packs, each with a single-use syringe | |
| 3.0 mL of HYMOVIS® in a 5.0-mL single-use syringe | |
| A hyaluronan concentration of 8 mg/mL, dissolved in physiologic saline | |
 |
| J-Code: J7322 | |
| CPT 20610; 20611 physician administration | |
 |
| Intra-articular hyaluronans are covered by most insurance plans | |
| For reimbursement assistance, please call 1-866-HYMOVIS (1-866-496-6847) | |
| For patient insurance verification on the web register/login to: www.fidiacomplete.com | |
| Reimbursement Guide | |
| Prior Authorization Checklist 2017 | |
| Tips for Clean Claims Submission | |
| Patient Enrollment Form | |
| Coding information for HYMOVIS | |
| Overview of Reimbursement Support | |
| Samples CMS 1450 Form for HYMOVIS | |
| Samples CMS 1500 Form for HYMOVIS | |
| Strategies to Appeal Denied Claims |
| Available 9 AM to 8 PM ET, Monday through Friday | |||||||||
| Hotline: 1-866-HYMOVIS (1-866-496-6847) | |||||||||
Dedicated reimbursement support including:
|
|||||||||
| Simple ordering process | |||||||||
| Report adverse event or product complaints | |||||||||
| For Customer Service, please email us at customerservice@fidiapharma.us | |||||||||
| For product-related specific information, please contact the Medical Office at medicaloffice@fidiapharma.us | |||||||||
| For additional information please email us at hymovis@fidiapharma.us |
Fidia offers COMPREHENSIVE SUPPORT
for your patients and your practice
The HYMOVIS® Support Hotline*: 1-866-HYMOVIS (1-866-496-6847)
or www.HYMOVISonline.com
| • | Available 9 AM to 8 PM ET, Monday through Friday | ||||||||||
| • | Dedicated reimbursement support | ||||||||||
| • | Three ways to enroll: phone, fax, or web
|
||||||||||
| • | Simplified ordering, billing, and return processing | ||||||||||
| • | Report adverse events or a product quality complaint | ||||||||||
| • | Contact sales representative to order posters or brochures | ||||||||||
| • | Medical information |
| * | The Hotline does not file claims for callers, nor can it guarantee that you will be successful in obtaining reimbursement. Third-party payment for medical products and services is affected by numerous factors, not all of which can be anticipated or solved by the Hotline. |
FIDIA offers
|
Fidia offers COMPREHENSIVE SUPPORT for your patients and your practice The HYMOVIS® Support Hotline*: 1-866-HYMOVIS (1-866-496-6847) or www.HYMOVISonline.com
|
| No minimum order quantity | |
| Low thresholds for contract pricing | |
| Easy online ordering | |
| Each carton contains two blister packs, each with a single-use syringe | |
| 3.0 mL of HYMOVIS® in a 5.0-mL single-use syringe | |
| A hyaluronan concentration of 8 mg/mL, dissolved in physiologic saline | |
 |
| J-Code: J7322 | |
| CPT 20610; 20611 physician administration | |
 |
| Intra-articular hyaluronans are covered by most insurance plans | |
| For reimbursement assistance, please call 1-866-HYMOVIS (1-866-496-6847) | |
| For patient insurance verification on the web register/login to: www.fidiacomplete.com | |
| Reimbursement Guide | |
| Prior Authorization Checklist 2017 | |
| Tips for Clean Claims Submission | |
| Patient Enrollment Form | |
| Coding information for HYMOVIS® | |
| Overview of Reimbursement Support | |
| Samples CMS 1450 Form for HYMOVIS® | |
| Samples CMS 1500 Form for HYMOVIS® | |
| Strategies to Appeal Denied Claims |
| Available 9 AM to 8 PM ET, Monday through Friday | |||||||||
| Hotline: 1-866-HYMOVIS (1-866-496-6847) | |||||||||
Dedicated reimbursement support including:
|
|||||||||
| Simple ordering process | |||||||||
| Report adverse event or product complaints | |||||||||
| For Customer Service, please email us at customerservice@fidiapharma.us | |||||||||
| For product-related specific information, please contact the Medical Office at medicaloffice@fidiapharma.us | |||||||||
| For additional information please email us at hymovis@fidiapharma.us |
*Mechanical testing may not be indicative of human clinical outcomes
** From a multi-center open label study in 49 patients treated with HYMOVIS®