
S. Bisicchia, G. Bernardi, C. Tudisco
Hymovis (HYADD®4, Fidia Farmaceutici Spa, Abano Terme, Italy) is a new hydrogel HA-based product, which is a new hyaluronan (HA)-based highly viscoelastic hydrogel for the treatment of osteoarthritis knee pain, which has improved shock absorbing and lubricating properties.*
This is a single-center, single-blind, prospective randomized controlled clinical study to compare clinical results and quality of life in patients with symptomatic knee osteoarthritis randomized to either Hymovis® or corticosteroid (CS). An additional analysis was conducted for safety and tolerability.
Patients were evaluated at baseline, 6, 12, 26 and 52 week post-injection.
Primary end point: WOMAC total score at 26 weeks;
Secondary end points: WOMAC total score, VAS for pain, and SF-36 score at any time point.
Results
Hymovis® clinical performance
While patients in both groups obtained good results in the short term (6 weeks), patients in the Hymovis® group obtained better results at 26 weeks in terms of knee function, WOMAC total score (p<0.0001), pain VAS (p=0.004) and quality of life.
HYMOVIS®-treated patients had significantly greater improvement in total WOMAC scores, VAS for pain scores, and SF-36 scores at 26 weeks
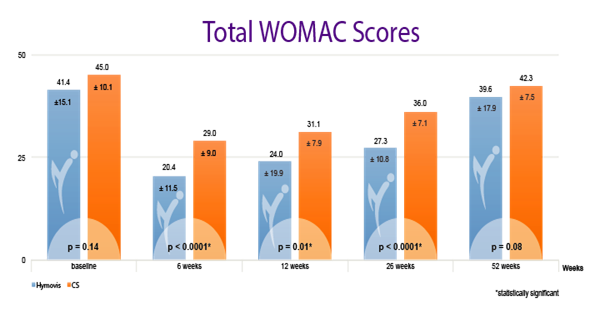
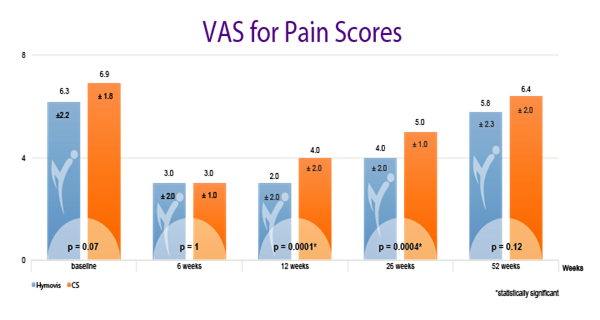
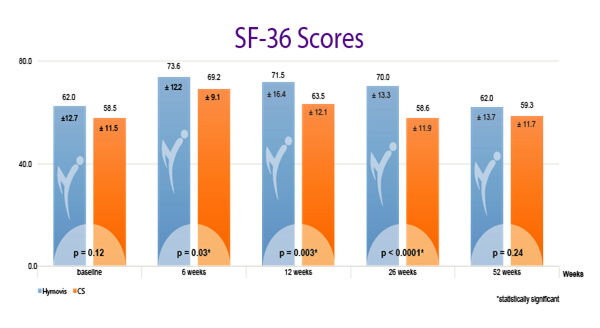
Efficacy outcomes in patients treated with 2 weekly IA injections of either Hymovis (HYADD®4) or corticosteroid: Total WOMAC Scores; VAS for Pain Scores; and quality of life as shown by patient responses on the SF-36 questionnaire.
*Data on file

|
Fidia offers COMPREHENSIVE SUPPORT for your patients and your practice The HYMOVIS Support Hotline*: 1-866-HYMOVIS (1-866-496-6847) or www.HYMOVISonline.com
|
| No minimum order quantity | |
| Low thresholds for contract pricing | |
| Easy online ordering | |
| Each carton contains two blister packs, each with a single-use syringe | |
| 3.0 mL of HYMOVIS® in a 5.0-mL single-use syringe | |
| A hyaluronan concentration of 8 mg/mL, dissolved in physiologic saline | |
 |
| J-Code: J7322 | |
| CPT 20610; 20611 physician administration | |
 |
| Intra-articular hyaluronans are covered by most insurance plans | |
| For reimbursement assistance, please call 1-866-HYMOVIS (1-866-496-6847) | |
| For patient insurance verification on the web register/login to: www.fidiacomplete.com | |
| Reimbursement Guide | |
| Prior Authorization Checklist 2017 | |
| Tips for Clean Claims Submission | |
| Patient Enrollment Form | |
| Coding information for HYMOVIS | |
| Overview of Reimbursement Support | |
| Samples CMS 1450 Form for HYMOVIS | |
| Samples CMS 1500 Form for HYMOVIS | |
| Strategies to Appeal Denied Claims |
| Available 9 AM to 8 PM ET, Monday through Friday | |||||||||
| Hotline: 1-866-HYMOVIS (1-866-496-6847) | |||||||||
Dedicated reimbursement support including:
|
|||||||||
| Simple ordering process | |||||||||
| Report adverse event or product complaints | |||||||||
| For Customer Service, please email us at customerservice@fidiapharma.us | |||||||||
| For product-related specific information, please contact the Medical Office at medicaloffice@fidiapharma.us | |||||||||
| For additional information please email us at hymovis@fidiapharma.us |
Fidia offers COMPREHENSIVE SUPPORT
for your patients and your practice
The HYMOVIS Support Hotline*: 1-866-HYMOVIS (1-866-496-6847)
or www.HYMOVISonline.com
| • | Available 9 AM to 8 PM ET, Monday through Friday | ||||||||||
| • | Dedicated reimbursement support | ||||||||||
| • | Three ways to enroll: phone, fax, or web
|
||||||||||
| • | Simplified ordering, billing, and return processing | ||||||||||
| • | Report adverse events or a product quality complaint | ||||||||||
| • | Contact sales representative to order posters or brochures | ||||||||||
| • | Medical information |
| * | The Hotline does not file claims for callers, nor can it guarantee that you will be successful in obtaining reimbursement. Third-party payment for medical products and services is affected by numerous factors, not all of which can be anticipated or solved by the Hotline. |
FIDIA offers
|
Fidia offers COMPREHENSIVE SUPPORT for your patients and your practice The HYMOVIS Support Hotline*: 1-866-HYMOVIS (1-866-496-6847) or www.HYMOVISonline.com
|
| No minimum order quantity | |
| Low thresholds for contract pricing | |
| Easy online ordering | |
| Each carton contains two blister packs, each with a single-use syringe | |
| 3.0 mL of HYMOVIS® in a 5.0-mL single-use syringe | |
| A hyaluronan concentration of 8 mg/mL, dissolved in physiologic saline | |
 |
| J-Code: J7322 | |
| CPT 20610; 20611 physician administration | |
 |
| Intra-articular hyaluronans are covered by most insurance plans | |
| For reimbursement assistance, please call 1-866-HYMOVIS (1-866-496-6847) | |
| For patient insurance verification on the web register/login to: www.fidiacomplete.com | |
| Reimbursement Guide | |
| Prior Authorization Checklist 2017 | |
| Tips for Clean Claims Submission | |
| Patient Enrollment Form | |
| Coding information for HYMOVIS | |
| Overview of Reimbursement Support | |
| Samples CMS 1450 Form for HYMOVIS | |
| Samples CMS 1500 Form for HYMOVIS | |
| Strategies to Appeal Denied Claims |
| Available 9 AM to 8 PM ET, Monday through Friday | |||||||||
| Hotline: 1-866-HYMOVIS (1-866-496-6847) | |||||||||
Dedicated reimbursement support including:
|
|||||||||
| Simple ordering process | |||||||||
| Report adverse event or product complaints | |||||||||
| For Customer Service, please email us at customerservice@fidiapharma.us | |||||||||
| For product-related specific information, please contact the Medical Office at medicaloffice@fidiapharma.us | |||||||||
| For additional information please email us at hymovis@fidiapharma.us |
*Mechanical testing may not be indicative of human clinical outcomes
** From a multi-center open label study in 49 patients treated with HYMOVIS®