What other treatments are available for osteoarthritis?
If you have pain due to osteoarthritis of the knee, there are treatments that do not involve injections of HYMOVIS® into the joint. These include:
If you have pain due to osteoarthritis of the knee, there are treatments that do not involve injections of HYMOVIS® into the joint. These include:


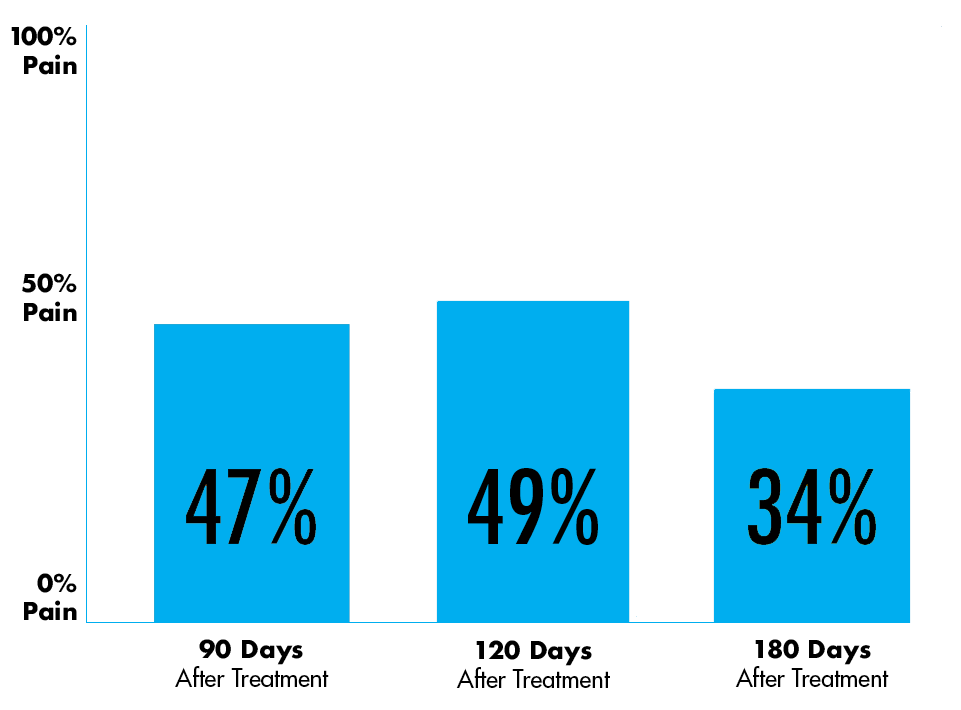
Patients in a HYMOVIS® Clinical Trial Experienced Clinical Benefit in Their Pain Reduction From Pre-Treatment Levels3.
Important Safety Information
HYMOVIS® is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy or simple analgesics (e.g., acetaminophen).
HYMOVIS® is contraindicated in patients with known hypersensitivity (allergy) to hyaluronate preparations or gram-positive bacterial proteins. Do not administer HYMOVIS® to patients with infections or skin diseases in the area of the injection site or joint.
The safety and effectiveness of the use of HYMOVIS® have not been tested in pregnant women, nursing mothers, or children. The safety and effectiveness of the use of HYMOVIS® in joints other than the knee, or for use concomitantly with other intra-articular (IA) injections, have not been established. The effectiveness of repeat treatment cycles of HYMOVIS® has not been established.
Arthralgia, transient pain, or swelling may occur after the IA injection. The incidence of arthralgia in the clinical study for HYMOVIS® was equivalent to the control group. No serious adverse events or pseudoseptic reactions were reported. Transient increases in inflammation following any IA hyaluronan injection have been reported in some patients with inflammatory joint conditions.
Strict aseptic technique should be used by licensed medical professionals trained to deliver agents into the knee joint. Joint effusion should be removed prior to injection of HYMOVIS®. Do not use disinfectants containing quaternary ammonium salts for skin preparation as hyaluronan can precipitate in their presence.
Patients should avoid strenuous or prolonged (eg, more than one hour) physical activities within 48 hours following the IA injection.
Please see Prescribing Information.
Rx Only
HYMOVIS and HYADD 4 are registered trademarks of Fidia Farmaceutici S.p.A., Abano Terme, Italy. HYMOVIS® is manufactured by Fidia Farmaceutici S.p.A., Abano Terme, Italy. ©2017 Fidia Pharma USA Inc, Florham Park, NJ 07054